Research Interests
The research interests of the Sarles group are inspired by the stimuli-responsive multi-functionality and selective transport of biomolecules, cellular membranes, and biological tissues. Our research capabilities and projects aim to advance our ability to assemble, characterize, and engineer functional materials based on these systems.
Assembly and characterization of biomimetic membranes
The Sarles group has extensive research experience in the assembly and characterization of lipid- and polymer-based biomimetic membranes assembled at liquid interfaces using the droplet interface bilayer technique. We have developed methods specifically relevant to the proposed work for assembling and stabilizing model cell membranes from synthetic and natural phospholipids, devised measurement techniques for assessing the thickness and lateral tension of planar biomembranes, and developed an automated, microfluidic membrane formation platform that enables the formation of model membranes with asymmetric leaflet compositions and which features integrated electrodes for precise measurements of membrane capacitance and transmembrane ion currents. Together, these tools and advances offer new opportunities for scientists to easy create and more quantitatively study model membranes.

Representative publications
- Venkatesan, et al, Evaporation-induced monolayer compression improves droplet interface bilayer formation using unsaturated lipids. Biomicrofluidics 2018, 12 (2), 024101.
- Taylor, et al, Capacitive Detection of Low-Enthalpy, Higher-Order Phase Transitions in Synthetic and Natural Composition Lipid Membranes. Langmuir 2017, 33 (38), 10016-10026.
- Nguyen, et al, Hydrodynamic trapping for rapid assembly and in situ electrical characterization of droplet interface bilayer arrays. Lab on a chip 2016, 16 (18), 3576-3588.
- Tamaddoni, et al, Reversible, voltage-activated formation of biomimetic membranes between triblock copolymer-coated aqueous droplets in good solvents. Soft Matter 2016, 12, 5096 – 5109.
- Venkatesan, G. A.; Sarles, S. A., Droplet immobilization within a polymeric organogel improves lipid bilayer durability and portability. Lab on a Chip 2016, 16, 2116 – 2125.
- Taylor, et al, Direct in situ measurement of specific capacitance, monolayer tension, and bilayer tension in a droplet interface bilayer. Soft Matter 2015, 11 (38), 7592-7605.
- Taylor, G. J.; Sarles, S. A., Heating-Enabled Formation of Droplet Interface Bilayers Using Escherichia coli Total Lipid Extract. Langmuir 2015, 31 (1), 325-337.
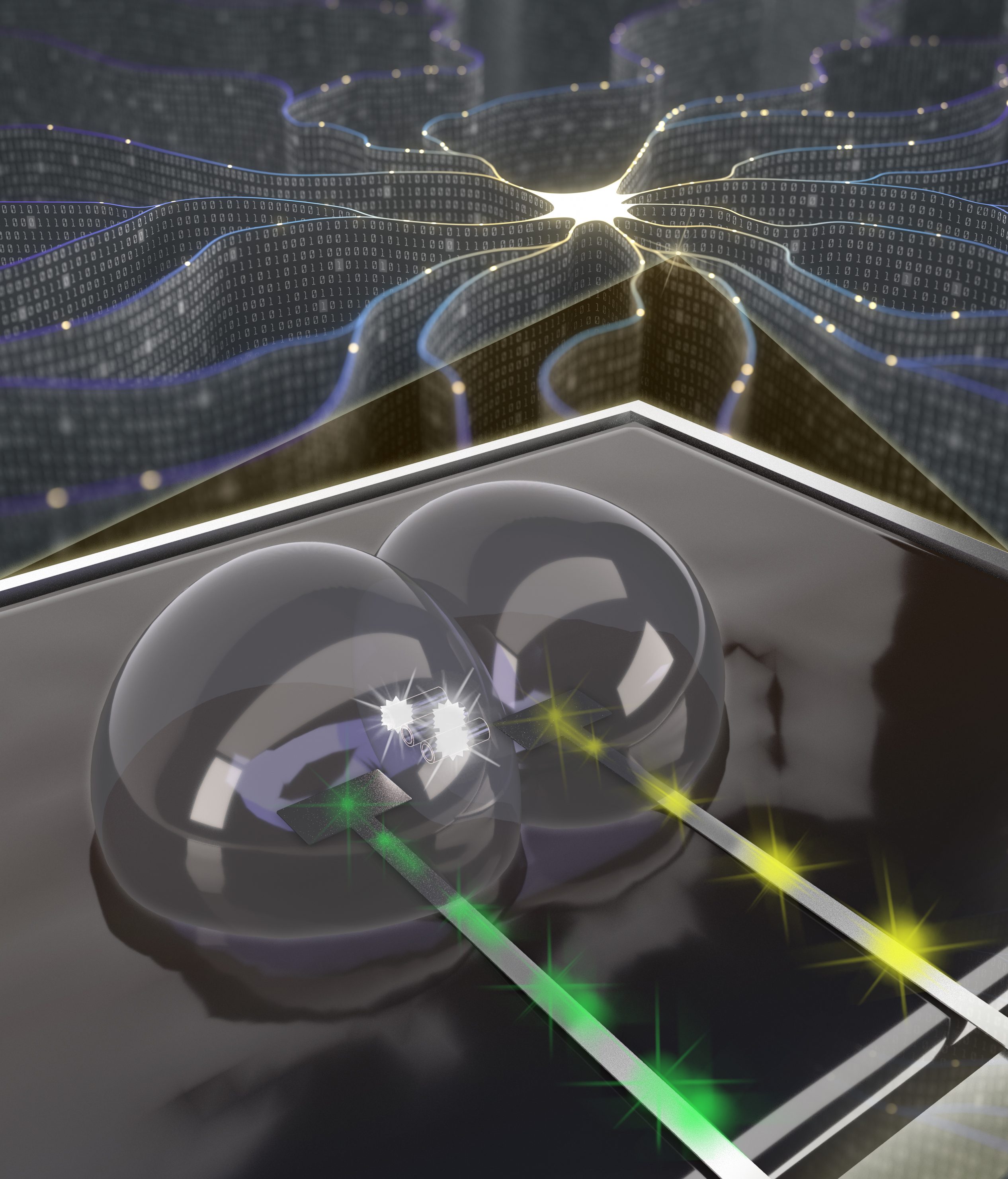
Reconfigurable materials for neuromorphic multifunctionality
The peripheral and central nervous systems in living animals comprise complex networks of distributed sensory and actuation elements and learning and memory centers (i.e. synapses and neurons) that allow animals to perceive, react, adapt to, and remember a wide variety of external stimulations, a collection of functions that man-made material systems do not offer. To address this gap, the Sarles group is researching the use of soft, reconfigurable materials for developing memory computing elements that mimic the functions of synapses and neurons in the brain. Significant contributions to date include a fundamentally new type of soft, biomolecular neuromorphic computing hardware that exhibits memory-resistance. Unlike other memristors developed in recent years, this embodiment uniquely features similar structure (biomembrane), switching mechanism (voltage-gated ion channels), and ionic transport modality as biological synapses and operates at considerably lower-power (0.1-1.0nW) than prior solid-state memristors.
Representative publications
- Najem, et al, Synapse-inspired variable conductance in biomembranes: a preliminary study. Proc. ASME 2017, SMASIS2017-3820, V001T08A005.
- Najem, et al, Memristive Ion Channel-Doped Biomembranes as Synaptic Mimics. ACS Nano 2018, 12 (5), 4702-4711.
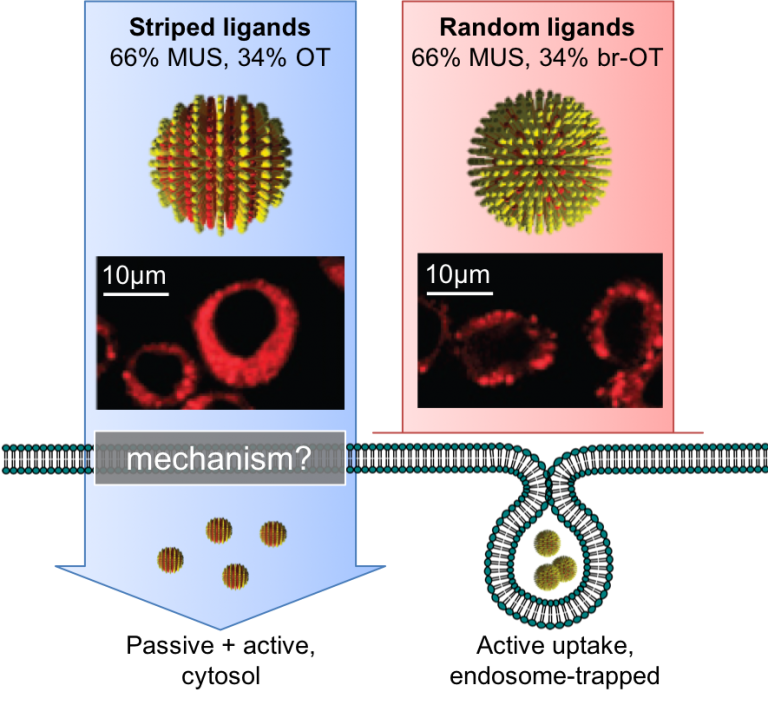
Nanomaterial-membrane interactions
While several types of amphiphilic synthetic nanoparticles (NPs) have shown to non-disruptively penetrate the plasma membranes of cells, the biophysical mechanism(s) of entry and the roles of membrane composition and lateral organization on NP uptake remain poorly understood. These gaps are attributed to insufficient techniques for assessing insertion and translocation and model membranes that to-date have failed to capture the complexity of real membranes. The Sarles group is actively collaborating with Prof. Francesco Stellacci at EPFL to understand of how striped amphiphilic NPs produced by the Stellacci group affect biomembrane interfaces by pioneering new techniques to quantify these nanoscale interactions using model membranes.
Representative publications
- Taylor, et al, Direct in situ measurement of specific capacitance, monolayer tension, and bilayer tension in a droplet interface bilayer. Soft matter 2015, 11 (38), 7592-7605.
- Taylor, G. J.; Sarles, S. A., Heating-Enabled Formation of Droplet Interface Bilayers Using Escherichia coli Total Lipid Extract. Langmuir 2015, 31 (1), 325-337.
Sponsors
We are grateful for financial support of our work from the following current and past sponsors:
